TIM-3 Therapy for Alzheimer’s: A New Hope for Memory Restoration
TIM-3 therapy for Alzheimer’s is an innovative approach that leverages our understanding of the immune system to combat this devastating disease. Recent studies suggest that inhibiting the TIM-3 checkpoint molecule may empower microglia, the brain’s immune cells, to clear amyloid plaques associated with Alzheimer’s. By enhancing the ability of these cells to address neuroinflammation and restore cognitive function, TIM-3 therapy could represent a turning point in Alzheimer’s therapy. Promising research has shown improved memory in mice treated with this method, signifying a potential shift in treatment paradigms, similar to strategies already used in cancer treatment. As scientists explore the links between immune response and Alzheimer’s pathology, TIM-3 emerges as a crucial player in potentially altering the course of this neurodegenerative disease.
Exploring novel treatments for Alzheimer’s disease has led researchers to consider checkpoint inhibitors previously used in cancer immunotherapy. By focusing on the TIM-3 molecule, scientists aim to reactivate microglial function to efficiently tackle neuroinflammation and clear harmful amyloid plaques from the brain. These microglia, which play a vital role in maintaining brain health, have become sidelined due to their excessive expression of TIM-3, leading to the accumulation of plaques and subsequent cognitive decline. Innovations in immune modulation, particularly targeting TIM-3, could offer exciting new avenues for Alzheimer’s therapy, potentially restoring memory and cognitive function. As we learn more about the interplay between immune regulation and neurodegenerative diseases, such strategies may redefine our approach to treating Alzheimer’s.
The Promise of TIM-3 Therapy for Alzheimer’s Disease
Recent advancements in Alzheimer’s therapy have highlighted the potential of TIM-3 therapy as a transformative approach to treating the disease. Research led by Dr. Vijay Kuchroo and his team uncovered that the TIM-3 checkpoint molecule, typically used in cancer treatment, could play a crucial role in addressing the plaque buildup associated with Alzheimer’s. By inhibiting TIM-3 expression in microglia, the brain’s immune cells, researchers found that these cells became more effective at targeting and eliminating amyloid plaques, which are characteristic of Alzheimer’s disease. This newfound understanding suggests that TIM-3 therapy not only aims to clear existing plaques but also potentially restores cognitive functionality in affected individuals, offering hope where traditional therapies have faltered.
The implications of TIM-3 therapy extend beyond just plaque clearance; they touch on the complex interplay of neuroinflammation and immune responses in the brain. In typical Alzheimer’s pathology, the accumulation of amyloid-beta plaques incites a neuroinflammatory response where microglia become hyperactivated, yet remain ineffective due to TIM-3 overexpression. By utilizing anti-TIM-3 therapies, it may be possible to enhance the microglia’s natural functions, promoting better synaptic health and memory retention in patients suffering from severe neurodegeneration. As researchers explore these innovative strategies, TIM-3 therapy stands poised as a potential pivotal advancement in the landscape of Alzheimer’s intervention.
Understanding Microglia and Their Role in Alzheimer’s
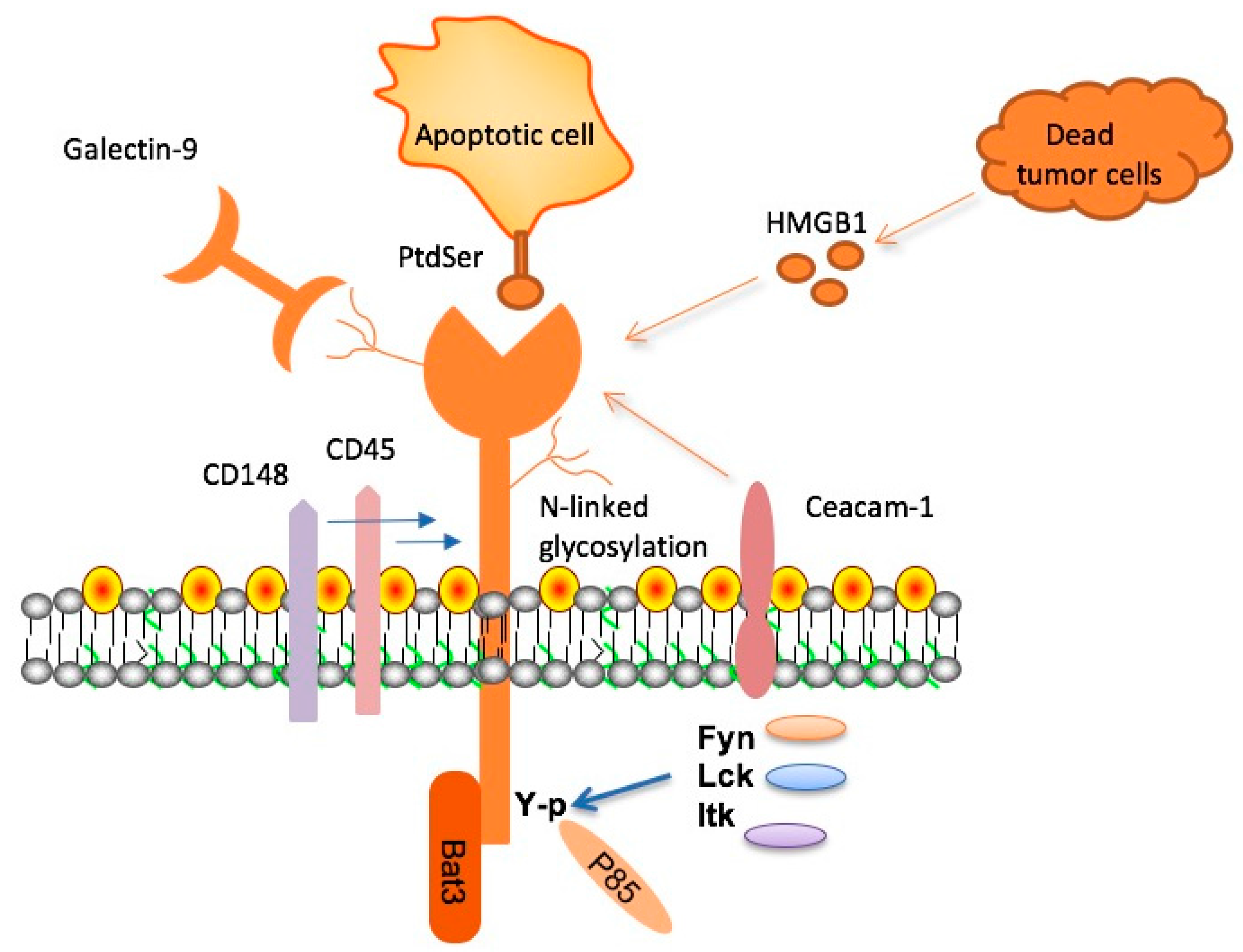
Microglia are often referred to as the brain’s immune system, taking on essential roles in maintaining neural health and functionality. In the context of Alzheimer’s disease, these cells are crucial as they are responsible for clearing out pathological elements like amyloid plaques. However, with aging and the onset of disease, microglia can become dysfunctional and fail to perform their clearing duties effectively. The overexpression of inhibitory molecules such as TIM-3 compromises their ability to engage with and eliminate harmful plaques. This dysfunction contributes significantly to the progression of Alzheimer’s by allowing plaques to accumulate, exacerbating neuroinflammation, and impairing the brain’s cognitive capacities.
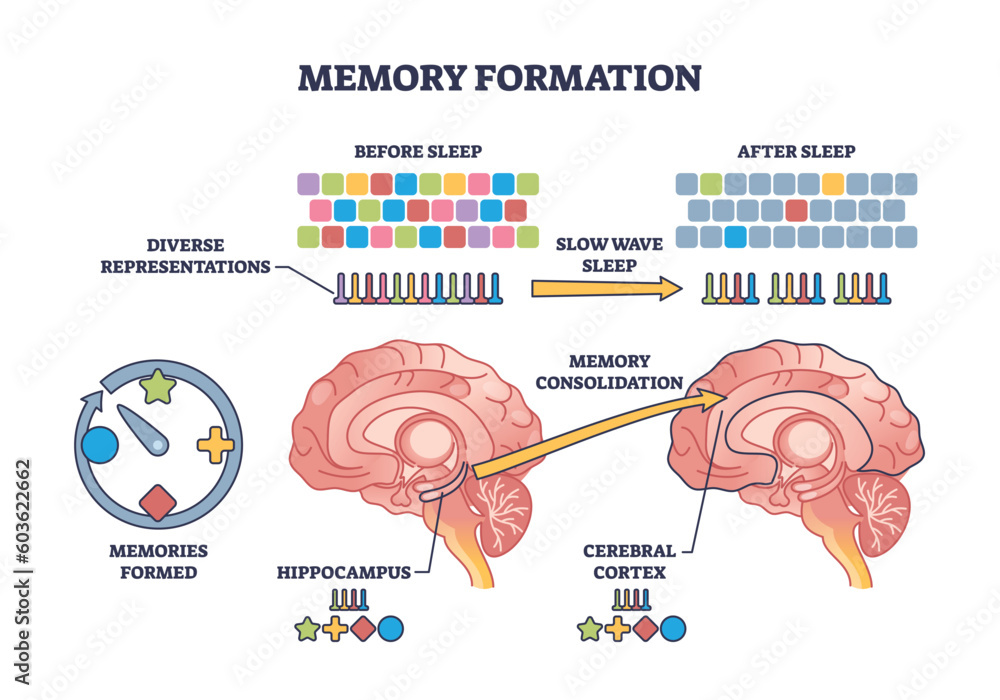
The impact of microglial dysfunction is particularly pronounced in late-onset Alzheimer’s, which comprises 90 to 95 percent of AD cases. In healthy brains, microglia engage in synaptic pruning, a process vital for memory formation and retention. However, under abnormal conditions, such as those witnessed in Alzheimer’s, microglia lose this ability and instead contribute to chronic inflammation. By reactivating these immune cells through targeted therapies that inhibit TIM-3 expression, researchers hope to restore their capacity to clear plaques effectively, which could lead to improvements in cognitive function and memory in affected individuals.
Neuroinflammation: The Link Between Alzheimer’s and TIM-3
Neuroinflammation plays a critical role in the pathogenesis of Alzheimer’s disease, creating a detrimental cycle that perpetuates cellular dysfunction and plaque accumulation. The presence of amyloid-beta plaques triggers an inflammatory response from microglia, which, when hampered by checkpoint molecules like TIM-3, leads to a state of exhaustion rather than effective clearance. This phenomenon posits TIM-3 as a potential therapeutic target, whereby reducing its expression could invigorate microglial activity against plaques, thereby alleviating neuroinflammation and its associated effects such as memory loss and cognitive decline.
In investigating neuroinflammation’s relationship with TIM-3, recent studies suggest that reducing TIM-3 expression could help recalibrate the microglial response, allowing them to re-engage in their normal immunological functions. If TIM-3 therapy can successfully rejuvenate the impaired immune activity in the brains of Alzheimer’s patients, it may not only improve plaque clearance but also dampen the overall inflammatory response. This dual action of clearing plaques while moderating neuroinflammation represents a promising avenue of research that intertwines findings from cancer treatment strategies with those aimed at combating neurodegenerative diseases.
Repurposing Cancer Therapies for Alzheimer’s Treatment
The concept of repurposing existing therapies from the field of cancer treatment for Alzheimer’s disease is gaining traction in medical research. Given that TIM-3 has been effectively targeted in cancer immunotherapy, utilizing similar antibodies or small molecules to inhibit its function in microglia presents a novel approach to Alzheimer’s therapy. The potential for these therapies lies in their ability to leverage established immunological principles that have proven successful in oncology, transforming their application to tackle neurodegenerative processes, thereby broadening the scope of effective treatments available for Alzheimer’s disease.
This innovative approach underscores the importance of cross-disciplinary research, as lessons learned from oncology can inform neuroimmunological strategies. By developing anti-TIM-3 antibodies or small molecules that leverage the immune checkpoints typically involved in tumor immunity, researchers hope to initiate a paradigm shift in Alzheimer’s treatment. This transformation could lead to substantial advancements in treatment efficacy, particularly for late-onset Alzheimer’s patients who may benefit from therapies designed to restore normal microglial function and memory.
Future Directions in Alzheimer’s Research
Looking ahead, the field of Alzheimer’s research is brimming with potential, particularly with the ongoing studies that aim to unravel the complexities of TIM-3 therapy. Future directions include exploring various formulations of TIM-3 inhibitors, testing their effectiveness in different stages of Alzheimer’s disease, and assessing their safety profiles in human subjects. The goal is not just to minimize plaque accumulation but also to enhance cognitive functioning and quality of life for those affected by Alzheimer’s.
Additionally, ongoing collaborations between institutions and researchers are crucial to the advancement of this field. As noted in recent findings, studies incorporating human TIM-3 models could provide a clearer understanding of how these therapies operate within the context of human biology. Such investigations will pave the way for clinical trials that are geared towards establishing a new standard of care in Alzheimer’s therapies, drawing from insights in both cancer treatments and neurobiology to offer hope for reclaiming lost cognitive abilities.
Exploring the Genetics of TIM-3 in Alzheimer’s Disease
Genetic factors play a pivotal role in the predisposition to Alzheimer’s disease, with the TIM-3 gene identified as a significant risk factor among late-onset cases. Understanding the genetic polymorphisms associated with TIM-3 not only illuminates pathways for targeted therapies but also enhances our comprehension of individual risk profiles. With around 90 to 95 percent of Alzheimer’s cases being late-onset, the ethnicity and genetic backgrounds of Alzheimer’s patients could yield insights into the efficacy of TIM-3 targeting strategies, allowing for personalized treatment approaches that leverage genetic predispositions.
Furthermore, deeper genetic analyses of TIM-3 may unveil additional insights into its interactions with other immune checkpoints and neuroinflammatory mediators. Such investigations could harness a wider understanding of how microglial receptors and other immune regulators interact in the context of Alzheimer’s pathology, leading to a multifaceted approach to treatment. By exploring these genetics alongside therapeutic interventions, the future of Alzheimer’s care could become increasingly tailored, improving outcomes for diverse patient populations.
The Interplay Between Alzheimer’s Pathology and Immune Checkpoint Molecules
The interplay between Alzheimer’s pathology and immune checkpoint molecules like TIM-3 highlights a complex narrative of brain health and disease progression. As the microglia face the challenge of clearing amyloid plaques, their functionality can be hampered by an overabundance of inhibitory signals, preventing them from mounting an effective immune response. This relationship suggests that strategically targeting these checkpoint pathways could restore balance within the brain’s immune defenses, effectively allowing the brain to mount a more robust response against accumulating neurotoxic elements.
Current clinical research is investigating how well these immune checkpoint molecules can be modulated to enhance cognition and memory retention. By devising ways to selectively inhibit TIM-3 activity, researchers aim to refine the therapeutic window, allowing microglia to re-engage and efficiently clear plaques. This innovative approach underscores the potential for bridging fragmented research findings across different domains, ultimately paving the way for a comprehensive strategy against Alzheimer’s and similarly debilitating neurodegenerative conditions.
Clinical Trials and the Future of TIM-3 Therapy
As research progresses, clinical trials focusing on TIM-3 therapy for Alzheimer’s disease will be pivotal in validating the efficacy of this novel approach. The journey towards human applications of TIM-3 inhibition requires meticulous planning and rigorous assessments to ascertain the safety and effectiveness of such treatments. Researchers are eagerly anticipating outcomes from ongoing studies that explore the therapeutic potential of anti-TIM-3 antibodies in models that closely mimic human Alzheimer’s pathology.
If successful, these trials could signal a new dawn in Alzheimer’s treatment, moving beyond symptomatic relief to truly restorative therapies that target the underlying disease mechanisms. This paradigm shift represents not only a beacon of hope for Alzheimer’s patients but also reflects the potential for integrating insights from various scientific disciplines to create comprehensive strategies aimed at tackling complex diseases.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s focuses on blocking the TIM-3 molecule, which inhibits microglia from clearing amyloid plaques in the brain. By deleting TIM-3 expression, microglia are freed to attack these harmful plaques, potentially restoring cognitive function in Alzheimer’s disease.
How does TIM-3 therapy relate to immune checkpoint therapy in cancer treatment?
TIM-3 is classified as an immune checkpoint molecule, originally explored in cancer treatment to prevent immune system exhaustion. By leveraging TIM-3 therapy for Alzheimer’s, researchers aim to re-enable microglia to combat neuroinflammation and degrade amyloid plaques, similar to how cancer therapies reinvigorate T cells.
What role do microglia play in Alzheimer’s therapy involving TIM-3?
Microglia are the brain’s immune cells responsible for clearing out debris and plaques. In TIM-3 therapy for Alzheimer’s, the goal is to disable TIM-3 so these cells can effectively remove amyloid beta plaques and mitigate neuroinflammation, alleviating the disease’s symptoms.
What evidence supports the effectiveness of TIM-3 therapy in Alzheimer’s disease?
Studies involving mice genetically modified to lack the TIM-3 gene showed that microglia became more effective at clearing plaques, leading to improved memory and cognitive function. These findings suggest that TIM-3 therapy could have a profound impact on Alzheimer’s treatment in humans.
Can TIM-3 therapy be used alongside other Alzheimer’s therapies?
Yes, TIM-3 therapy could potentially complement existing Alzheimer’s treatments by targeting the underlying neuroinflammation and plaque accumulation. This approach might enhance overall treatment efficacy and potentially overcome the limitations seen with other Alzheimer’s therapies focused solely on amyloid clearance.
What are the next steps in TIM-3 therapy research for Alzheimer’s?
Researchers are currently investigating the impact of human anti-TIM-3 antibodies in mouse models of Alzheimer’s. Successful trials could lead to new therapies that halt plaque development and improve outcomes for patients suffering from this devastating disease.
What is the significance of the TIM-3 gene polymorphism in Alzheimer’s patients?
Patients with a specific polymorphism in the TIM-3 gene (HAVCR2) often exhibit higher levels of TIM-3, which inhibits microglial activity. This increased expression is linked to the accumulation of amyloid plaques and offers insights into targeted interventions through TIM-3 therapy.
How does TIM-3 therapy address neuroinflammation in Alzheimer’s?
TIM-3 therapy targets the regulatory mechanisms of microglia that contribute to neuroinflammation in Alzheimer’s disease. By inhibiting TIM-3, microglia can restore their phagocytic function, reducing the inflammatory response associated with plaque accumulation.
What potential improvements can TIM-3 therapy offer over traditional Alzheimer’s treatments?
TIM-3 therapy offers a novel approach by directly enhancing the immune response to amyloid plaques, which may result in better cognitive outcomes compared to traditional therapies that focus primarily on reducing plaque levels without addressing microglial dysfunction.
What challenges exist in developing TIM-3 therapy for Alzheimer’s in humans?
Challenges include ensuring that anti-TIM-3 therapies selectively target plaques without adverse effects, such as unwanted immune responses or damage to healthy brain tissue. Additionally, understanding the timing of treatment initiation relative to disease progression is crucial for clinical success.
| Key Point | Details |
|---|---|
| Background on TIM-3 and Alzheimer’s | TIM-3 is an immune checkpoint molecule linked to late-onset Alzheimer’s, inhibiting microglia from clearing amyloid plaques in the brain. |
| Microglia and their Role | Microglia are the brain’s immune cells, crucial for synapse pruning and memory retention. Increased TIM-3 expression inhibits their function in older adults. |
| Experimental Findings | Deleting the TIM-3 gene in mice improved memory and cognition by enhancing microglial activity and plaque-clearance. |
| Potential Human Therapy | An anti-TIM-3 antibody or small molecules could be used to block TIM-3’s inhibitory effects, potentially leading to better cognitive outcomes in Alzheimer’s patients. |
| Research Collaboration | The study took five years and involved collaboration among multiple researchers from two specialized labs. |
Summary
TIM-3 therapy for Alzheimer’s represents a promising new frontier in the treatment of this devastating disease. By targeting the TIM-3 checkpoint molecule, researchers have opened up avenues to enhance the immune response against amyloid plaques in the brain, leading to improved cognitive function in experimental models. The approach aims to harness the body’s own immune cells, microglia, to maintain brain health and memory. As studies progress, this innovative strategy could potentially redefine Alzheimer’s therapy, offering hope to millions affected by this condition.