Clinical Research Safety: Impact of Funding Cuts
Clinical research safety is a paramount concern in the medical field, as it directly pertains to the well-being of participants engaged in studies. Effective oversight through Institutional Review Boards (IRBs) ensures that patient safety in research is prioritized, safeguarding participants from potential risks associated with new treatments or procedures. However, recent funding challenges, such as those faced by Harvard Catalyst IRB due to federal grant freezes, threaten the infrastructure that underpins these critical safety measures. Without adequate NIH research funding, the ability to protect the rights and welfare of research volunteers may be compromised, leading to potential setbacks in medical advancements. Therefore, maintaining robust financial support for clinical research oversight is essential for fostering a safe environment for all involved and enhancing trust in the research process.
In the realm of medical research, the term ‘patient welfare protection’ underscores the importance of ensuring that individuals participating in studies are shielded from harm and unethical practices. This concept is intimately linked to the regulatory domain known as medical study oversight, which encompasses the evaluation and continuous monitoring of research endeavors. The challenges surrounding IRB funding, particularly within contexts like the Harvard Catalyst IRB, highlight the critical need for financial resources to sustain ethical standards in research. Furthermore, the influence of NIH funding on research safety illustrates how financial constraints can impede the essential mechanisms that maintain participant trust and safety in clinical trials. As such, the integrity of research is contingent upon robust support systems that prioritize both ethics and scientific advancement.
The Importance of Clinical Research Safety
Clinical research safety is paramount in ensuring that patients who participate in medical studies are protected from harm. Institutional Review Boards (IRBs) serve as the backbone of this protection by reviewing research proposals to ensure they meet ethical standards and regulations. Their oversight includes assessing risk factors, ensuring informed consent, and monitoring any adverse events that may arise during the study. This rigorous scrutiny is essential not only to safeguard participants but also to maintain public trust in the research community.
Without adequate clinical research safety measures, the potential for harm increases significantly. Historical instances of unethical research practices have unveiled the dire consequences of insufficient oversight, underscoring the need for stringent regulations. Today, the role of IRBs has evolved, allowing them to adapt to diverse research methodologies and settings while ensuring the safety and welfare of participants is prioritized.
Impact of Funding Challenges on Patient Safety in Research
The halt in federal funding, particularly the recent freeze affecting over $2 billion in research grants at Harvard, poses a significant threat to patient safety in clinical research. Funding cuts impact the ability of IRBs to function efficiently, leading to potential delays in the review and approval of necessary studies that prioritize patient welfare. As funding becomes scarce, maintaining a robust oversight system can falter, ultimately affecting the integrity of research outcomes and patient safety.
Moreover, with fewer resources available, institutions may struggle to fulfill their obligations to monitor ongoing studies. This could leave participants vulnerable as researchers may cut corners or neglect rigorous safety protocols under financial constraints. The ripple effects of funding challenges extend beyond immediate patient safety; they jeopardize public trust in medical research as a whole, creating skepticism and reluctance among potential research participants.
Navigating NIH Research Funding Impact
National Institutes of Health (NIH) funding plays a crucial role in supporting the infrastructure that protects patients in research settings. With appropriate funding, researchers can ensure comprehensive oversight by IRBs, which is vital for compliance with ethical standards and regulatory guidelines. The allocation of NIH funds to strengthen research ethics and safety protocols ensures that institutions have the resources to conduct studies responsibly and transparently.
However, funding reductions can severely hinder the ability of research institutions to maintain these standards. For instance, the introduction of the single IRB (sIRB) requirement for multisite research aims to streamline oversight but requires adequate funding to be implemented effectively. Without sufficient NIH funding, ensuring rigorous oversight becomes challenging, potentially exposing patients to greater risks during studies and undermining the quality of research.
Understanding Medical Study Oversight
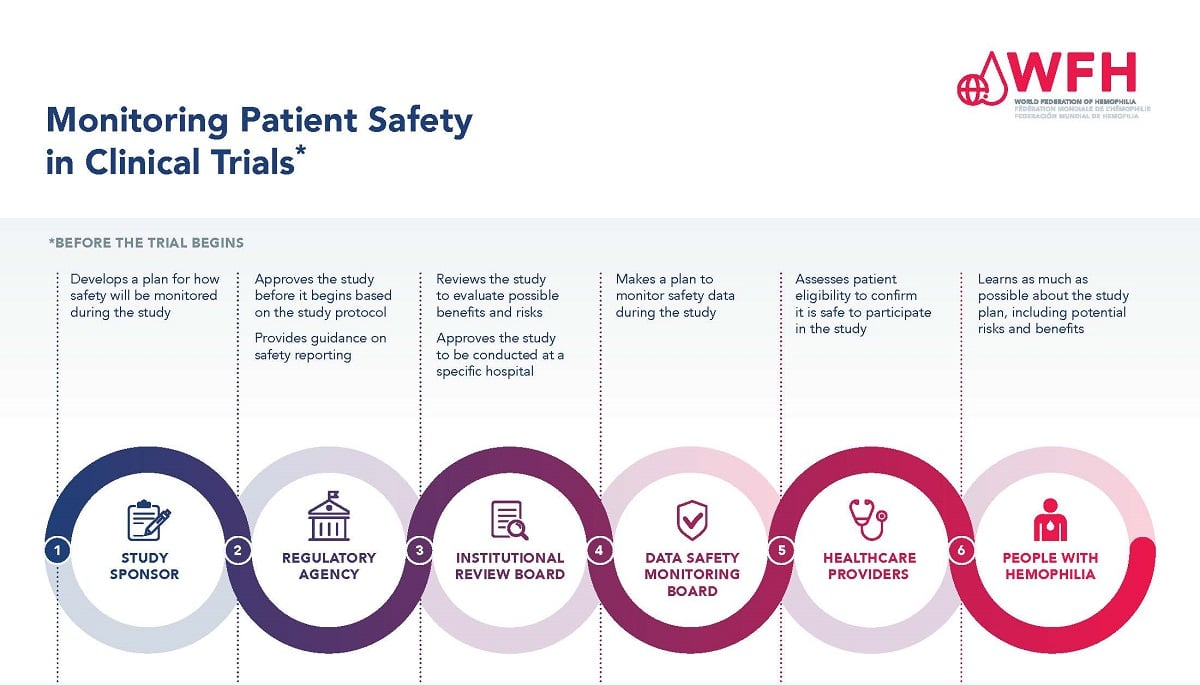
Medical study oversight is a vital process that extends beyond just ethical considerations—it’s an essential component of ensuring patient safety in clinical research. Oversight functions involve regular monitoring of ongoing studies, risk assessment, and the implementation of safety protocols that protect participants throughout the research lifecycle. With the support of institutional review boards and experienced personnel, medical study oversight brings a structured approach to managing risks associated with clinical trials.
As part of this framework, proper oversight contributes to building a collaborative research environment where institutions can share data and resources effectively. This teamwork not only streamlines the research process but also enhances the safety and welfare of participants involved. Particularly in studies that span multiple sites, robust oversight ensures consistent application of ethical standards and regulatory compliance across all participating institutions.
IRB Funding Challenges and Their Effects
Funding challenges faced by Institutional Review Boards (IRBs) can impede their ability to function adequately, ultimately impacting patient safety in research. As IRBs are often funded through institutional budgets, cuts can lead to understaffing, reduced review capacities, and longer wait times for study approvals. These issues could compromise the thoroughness of ethical reviews and oversight necessary for safeguarding research participants.
When IRBs operate under constrained budgets, the quality of oversight might suffer, leading to procedural shortcuts that may endanger patient safety. Moreover, diminished funding can affect the training and support provided to IRB staff, potentially resulting in a lack of awareness regarding emerging ethical challenges and regulatory changes that are critical to patient protection in research.
The Role of Harvard Catalyst IRB in Safety Assurance
Harvard Catalyst plays a pivotal role in ensuring that patient safety is prioritized in clinical research. As the driving force behind the SMART IRB system, it facilitates collaboration among institutions, ensuring that ethical reviews are streamlined and consistent across multiple sites. This collective effort diminishes administrative burdens, allowing researchers to focus more on designing safe and effective studies without compromising patient protections.
Moreover, Harvard Catalyst’s commitment to education and support for researchers enhances the ethical conduct of studies by equipping them with the necessary tools and knowledge to navigate complex regulatory environments. Effective collaboration in IRB review processes fosters a culture of accountability, ensuring that patient safety remains at the forefront of all medical research endeavors conducted under its auspices.
The Historical Context of Research Ethics
A thorough understanding of the historical context of research ethics is fundamental in comprehending the importance of patient safety in clinical studies. Historical abuses in research—such as the Tuskegee Study and other unethical experiments—have led to significant reforms necessitating direct oversight by IRBs to protect human subjects. These events have shaped regulations that provide a safety net for participants and highlight the critical need for ethics in research, which is still relevant today.
As a response to past transgressions, regulatory frameworks now require comprehensive safety protocols and informed consent processes that empower patients with knowledge about their rights and responsibilities. By studying these historical precedents, stakeholders in the research community can glean insights into preventing unethical practices and promoting patient safety in future medical studies.
Community Engagement and Research Transparency
Community engagement is becoming increasingly essential in the realm of clinical research, serving as a mechanism to enhance patient safety and trust. By actively involving community members in the research process, institutions can better understand participants’ perspectives and concerns, thereby creating more transparent and ethically sound research environments. Such collaboration helps align research objectives with community values, ensuring that studies do not occur in a vacuum but are tailored to meet the needs of those they aim to serve.
Moreover, transparent communication about study protocols, risks, and benefits fosters a sense of ownership among potential participants. When communities are informed and involved in research processes, they are more likely to support clinical studies while feeling confident that their safety and welfare are prioritized. This engagement not only enhances patient safety but also reinforces public trust, encouraging wider participation in future research endeavors.
The Future of Safe Clinical Research Practices
Looking ahead, the future of clinical research practices hinges on establishing and maintaining robust systems that prioritize patient safety. As the landscape of research evolves, integrating advanced technologies like artificial intelligence and data analytics can offer better monitoring and oversight capabilities. These innovations hold the potential to enhance patient safety by ensuring that research is conducted ethically and responsibly.
Additionally, as funding challenges continue to impact the research environment, establishing more sustainable funding models will be crucial to support IRBs and improve patient protection mechanisms. Ensuring that resources are allocated efficiently will reinforce the integrity of clinical research and help uphold the ethical standards essential for fostering public confidence in scientific endeavors. Ultimately, balancing innovation with rigorous safety protocols will be key to advancing medical research while safeguarding the interests of participants.
Frequently Asked Questions
How does clinical research safety protect patients during studies?
Clinical research safety is paramount as it ensures that patients participating in studies are safeguarded from harm. This is typically managed through rigorous oversight by Institutional Review Boards (IRBs) that review research proposals for ethical compliance, risk assessment, and informed consent procedures. By adhering to established guidelines, clinical research aims to protect the rights and welfare of all research participants.
What impact does NIH funding have on patient safety in research?
NIH funding plays a critical role in enhancing patient safety in research by supporting the establishment and functioning of IRBs. These boards conduct thorough reviews and oversight to ensure that all aspects of a study protect patients’ rights and well-being. The funding also promotes adherence to safety regulations, which is vital for maintaining public trust in clinical trials.
What are the challenges related to medical study oversight due to funding cuts?
Funding cuts can severely hinder medical study oversight, as they often lead to a reduction in resources available for IRB operations. This can result in delays in study approvals, inadequate oversight, and an increased risk of ethical violations, which ultimately jeopardizes patient safety and public confidence in the research process.
How does the interruption of Harvard Catalyst IRB funding affect clinical research safety?
The interruption of Harvard Catalyst IRB funding directly impacts clinical research safety by halting critical oversight functions. This disruption limits the ability to add new sites to ongoing studies, which can delay research trials and compromise patient safety measures that are essential for ethical medical research.
What preventive measures are taken to ensure patient safety in clinical research?
Preventive measures for ensuring patient safety in clinical research include comprehensive IRB review processes, thorough informed consent procedures, risk-benefit analysis, and continuous monitoring of adverse events throughout the study. These protocols are put in place to identify and mitigate potential risks to participants and maintain ethical standards.
How do IRB funding challenges impact the safety of patients involved in medical research?
IRB funding challenges create obstacles in maintaining proper oversight necessary for safeguarding patients in medical research. Insufficient funding can limit the IRB’s capacity to conduct thorough reviews, reducing their ability to address ethical concerns and ensuring that participants’ rights and safety are prioritized throughout the study.
What historical events influenced the development of clinical research safety regulations?
Historical events such as the Tuskegee Syphilis Study and the unethical treatment of subjects in medical experimentation highlight the critical need for robust clinical research safety regulations. These events led to the establishment of ethical guidelines and oversight frameworks, such as IRBs, to protect the rights and welfare of patients in clinical research.
How can patient safety in research be improved with better funding?
Improved funding for clinical research can enhance patient safety by ensuring that IRBs have adequate resources to conduct comprehensive studies. This includes better training for IRB members, increased capacity to monitor ongoing research, and the ability to implement rigorous safety protocols that protect participants, ultimately fostering trust in the research process.
| Key Point | Details |
|---|---|
| Funding Cuts Impact | Federal research grants cut off lead to patient safety risks in clinical studies. |
| Role of IRBs | Institutional Review Boards oversee compliance and ensure participant rights and welfare. |
| Watchdog Function | IRBs provide checks and balances for ethical medical research practices. |
| Historical Context | Past medical injustices fuel the need for strict oversight in research today. |
| Community Trust | Funding cuts threaten public trust and willingness to participate in research. |
| Continuing Support | Harvard Medical School’s support crucial for sustaining collaborative research. |
Summary
Clinical research safety is paramount to the integrity and effectiveness of medical studies. Recent cuts in funding threaten this critical area, impacting the rights and safety of patients involved in clinical research. Without proper oversight and resources, the ethical conduct of research suffers, jeopardizing patient well-being and public trust in medical innovation. It is essential for institutions to ensure ongoing support for safety practices to maintain a robust, ethical, and trusted clinical research landscape.