CRISPR Gene Editing: Ethical Questions and Promises
CRISPR gene editing represents a groundbreaking leap in biotechnology, promising the potential to alter the genetic foundations of life itself. With this innovative gene editing technology, researchers are exploring therapies for conditions like sickle cell anemia, transforming lives while navigating a complex landscape of ethical implications. The excitement surrounding CRISPR is tempered by serious discussions about the ethical dilemmas of gene modification, particularly as it relates to health equity in gene therapy. As scientists grapple with the implications of manipulating human genetics, questions emerge on who decides which traits to alter and the socio-economic factors that may disproportionately privilege certain populations. The balance between harnessing this powerful tool and addressing the moral challenges it presents is crucial for shaping the future of genetic medicine.
Gene editing, or gene modification, is at the forefront of scientific advancement, with CRISPR technology leading the charge in reimagining how we approach genetic disorders. This pioneering approach allows for precise alterations at the molecular level, opening doors to transformative treatments for diseases such as sickle cell anemia. However, alongside its potential benefits lie pressing issues regarding the moral ramifications of altering human DNA and the broader implications for societal health equity. As researchers delve into these complex matters, the conversation naturally veers towards the ethical ramifications of gene editing, raising critical questions about responsibility, access, and informed decision-making in therapeutic contexts. Engaging in these discussions is not only vital for the scientific community but also for society as a whole, as we collectively navigate this uncharted territory.
Understanding CRISPR Gene Editing and Its Potential
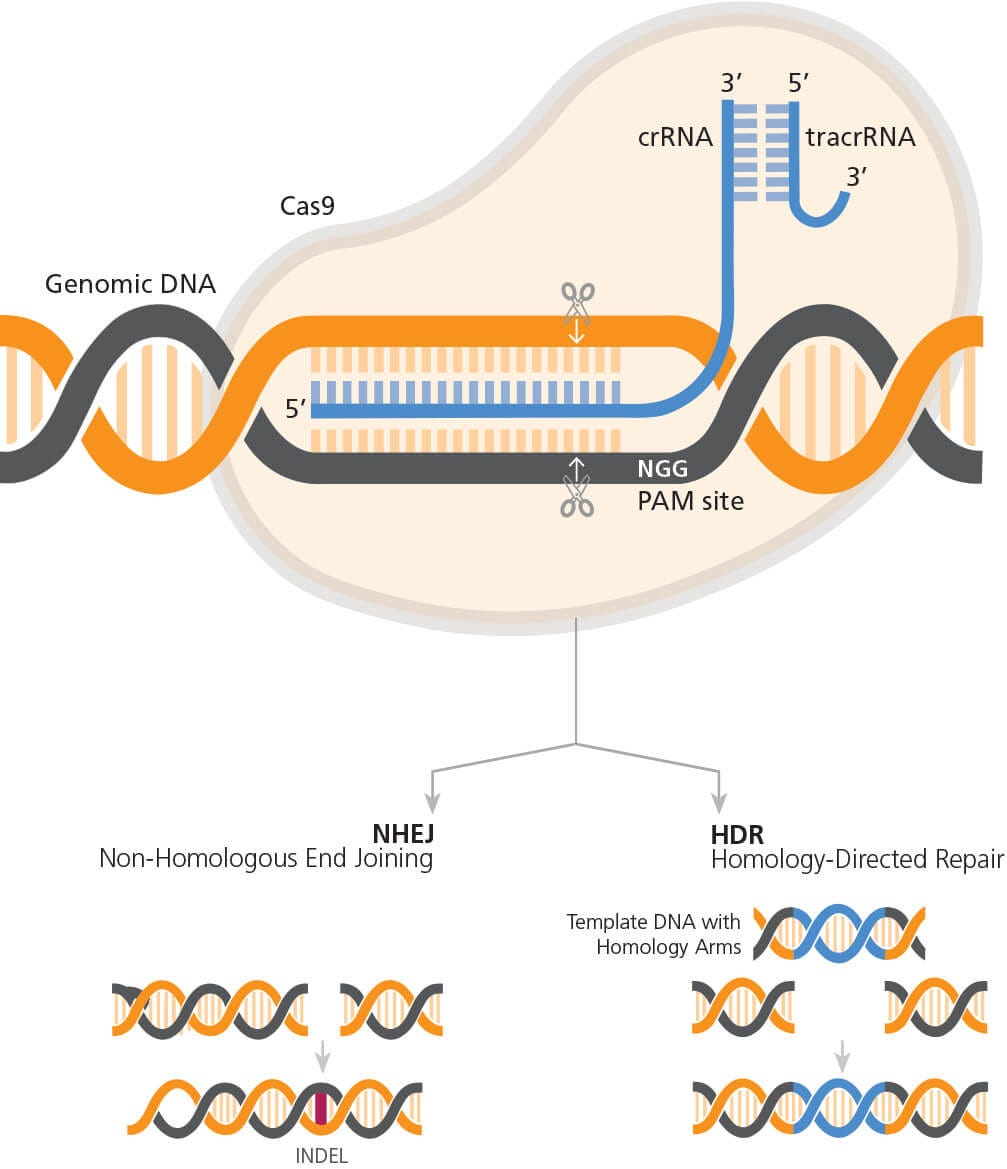
CRISPR gene editing stands at the forefront of modern science, providing a revolutionary method for altering DNA sequences within organisms. This technique offers the possibility of correcting genetic defects, preventing the spread of diseases, and advancing healthcare significantly. In particular, its application to treat conditions such as sickle cell anemia highlights the enormous potential of gene editing technology. As researchers continue to explore its applications, the prospect of curing genetic disorders becomes increasingly tangible.
However, the extraordinary capabilities of CRISPR commend us to carefully weigh its implications. The ease with which one can introduce genetic changes raises profound questions—are we prepared to handle the consequences of editing human life? For instance, while the idea of eradicating sickle cell anemia is enticing, the ethical implications surrounding the alteration of germline cells are daunting. The ability to manipulate hereditary traits leads to inevitable comparisons with eugenics, prompting discussions about societal norms and genetic privilege.
The Ethical Landscape of Gene Editing Technology
While CRISPR presents remarkable opportunities for scientific advancement, it also brings with it a host of ethical dilemmas that cannot be ignored. As discussed in recent forums, including those featuring experts like Neal Baer and Rebecca Brendel, the core question remains—does the ability to edit genes justify doing so? Ethical implications of gene editing include concerns about consent, especially in instances where genetic modifications might affect future generations who are unable to voice their agreement. Furthermore, factors such as class disparity in access to these technologies complicate the discussion, as modern innovation tends to cater primarily to affluent individuals.
During public talks, issues of health equity in gene therapy often emerge, emphasizing the disparity in access to state-of-the-art treatments. As Baer’s experience with sickle cell anemia evokes, the exorbitant cost of gene manipulation treatments creates a significant barrier for many families. This economic factor raises critical questions about who can afford these life-altering technologies, potentially exacerbating existing health inequalities. As society treads the path of genetic innovation, it must grapple with the moral responsibility to create equitable frameworks ensuring everyone can benefit from advancements in healthcare.
Curing Sickle Cell Anemia: A Double-Edged Sword
The advent of CRISPR technology has placed the potential cure for sickle cell anemia within reach, yet it is crucial to recognize that such advancements are not without their challenges. The prospect of eliminating a debilitating genetic disorder is an exciting breakthrough; however, it unveils a complex landscape of ethical considerations. Disability advocates argue that conditions like sickle cell are part of personal and cultural identities that should not simply be erased, shifting the focus from a purely medical condition to broader societal perceptions.
Moreover, the discussions highlighted by professionals underscore that while curing sickle cell is a noble goal, the implications surrounding gene editing must be addressed. Ethical ramifications arise from the decisions about which traits to modify or eliminate. As noted during these conversations, the possibility of editing out characteristics deemed undesirable—such as those associated with conditions compatible with life—requires serious moral reflection about our societal values and the motivations behind such choices. Ultimately, with great power comes great responsibility, demanding a nuanced approach to how we utilize CRISPR in medicine.
CRISPR Ethical Dilemmas: Who Decides What is ‘Normal’?
As we advance into the realm of CRISPR gene editing, one significant ethical dilemma surfaces—who gets to determine what constitutes ‘normal’? The idea of editing out conditions, such as hearing impairments or genetic disorders, leads to troubling implications about societal norms and acceptance of diversity. The perspective expressed by advocates like Carol Padden highlights the necessity of questioning whether certain traits are inherently ‘pathological’ or merely forms of human variation. This discourse is critical as it reframes our understanding of disability within the context of genetics.
The conversation shifts toward parental decision-making regarding genetic modifications for their children, raising moral considerations about autonomy and the potential for coercion. Ethical discussions emphasize that parents’ desires to alter their child’s genetic make-up should be carefully weighed against the implications these changes could have for individual identity and societal perceptions of diversity. As gene editing technology becomes increasingly sophisticated and obtainable, society must engage in broader dialogues about the values we prioritize in shaping future generations.
Global Impacts of Gene Editing: A Cautionary Tale
Despite the immense benefits that CRISPR gene editing can provide, its global implications pose significant concerns that must be carefully navigated. As highlighted by discussions on the ethical ramifications of gene editing, the ability to manipulate genetics extends beyond mere medical applications. In countries where regulations may be lax or non-existent, the potential for misuse or unethical experimentation increases. Reports suggest that access to CRISPR technology could lead to gene editing practices that prioritize certain populations over others, potentially creating a ‘genetic divide’ between developed and developing nations.
Additionally, the fear of unintended genetic consequences remains a topic rife with concern for scientists and ethicists alike. As Baer pointed out, changes to a single gene can have cascading effects that bioengineers or medical professionals may not fully anticipate. These risks require continuous monitoring and ethical scrutiny to prevent adverse outcomes while advocating for a responsible and equitable approach to the deployment of genetic editing applications. Such cautionary tales push for global cooperation in formulating comprehensive policy frameworks that ensure safety and fairness in the application of gene editing technologies.
Navigating Health Justice in Gene Therapy
The intersection of gene therapy and health justice requires thoughtful examination of how gene editing technologies are distributed and accessed globally. The inequities in healthcare are stark, with innovative treatments like CRISPR often being accessible only to those who can afford them. This raises pressing concerns about health equity, particularly for minority communities and economically disadvantaged populations who suffer disproportionately from genetic disorders like sickle cell anemia. Addressing health justice in gene therapy bears immediate relevance as it allows for a critical evaluation of systemic barriers that prevent equitable access to necessary treatments.
Professionals in bioethics argue for the inclusion of all stakeholders in discussions regarding gene editing technologies, advocating for policies that prioritize health equity. Incorporating diverse perspectives can lead to a better understanding of the social impact of these innovations, potentially fostering a more inclusive approach to medical advancements. As long as disparities remain, gene editing technologies might inadvertently perpetuate existing health inequalities rather than bridging the gap. Therefore, the integration of health justice principles into the development and distribution of CRISPR treatments is essential in creating a fairer healthcare ecosystem.
The Role of Oversight in Genetic Editing
Regulation and oversight of gene editing practices are essential in ensuring ethical compliance and public safety. As technological advancements in CRISPR evolve, the potential for unethical use increases, particularly in countries where legislative frameworks lag behind scientific developments. Without appropriate oversight, the ethical dilemmas associated with gene editing technologies can lead to significant public health risks and societal consequences. Stakeholders, including governmental bodies, scientists, and ethicists, must collaborate to create robust regulatory measures that monitor gene editing practices and ensure adherence to established ethical standards.
In addition to domestic regulations, global cooperation is necessary to establish norms that prevent reckless applications of gene editing technology. Collaborative international efforts can help understand the ethical boundaries of gene editing and prevent potentially harmful practices undertaken by individual nations. This global perspective can also facilitate a shared commitment to uphold moral standards, ensuring that CRISPR technologies are used responsibly and ethically—ultimately safeguarding humanity from the repercussions of uncontrolled genetic manipulation.
Potential Risks Associated with CRISPR Technology
While CRISPR offers unprecedented possibilities for genetic modification, it also carries potential risks that warrant serious consideration. The scientists’ push for rapid innovations can sometimes overlook the implications of gene editing on ecological balance and human health. As observed in recent advances, the process of altering genes, such as those regulating cholesterol levels, suggests that modifying a single gene could inadvertently affect multiple biological systems. This complexity highlights the urgent need for increased caution and thorough research before deploying gene editing technologies in clinical settings.
To mitigate such risks, an emphasis on comprehensive studies and ethical reviews is critical in guiding gene editing applications. Researchers and healthcare professionals are tasked with ensuring that the benefits of these advancements significantly outweigh potential hazards. Moreover, an informed public discourse surrounding the risks associated with CRISPR is essential to foster awareness and understanding, empowering communities to engage in discussions that shape the regulatory landscape of genetic editing technologies.
The Future of Genetic Editing: Hope or Hazard?
Looking ahead, the future of CRISPR gene editing teeters between the promise of groundbreaking breakthroughs and the specter of ethical complexities. As research accelerates and new applications are uncovered, society must grapple with the responsibility that comes along with such powerful genetic tools. The potential for crime, discrimination, and unintended consequences reminiscent of historical eugenics movements serves as a cautionary reminder of the socio-political dimensions that must accompany technological progress.
The ongoing discussions about the ethical implications of gene editing place tremendous emphasis on foresight, responsibility, and collective engagement. As promising as the advancements in CRISPR may appear, a vigilant and informed society—capable of engaging in robust ethical reasoning—is paramount to harnessing the power of gene editing in ways that contribute positively and equitably to human health. Healthy democratic debates surrounding such technologies can equip the scientists, policymakers, and the public with the necessary insights that guide responsible practices and protections for all.
Frequently Asked Questions
What are the ethical implications of CRISPR gene editing?
CRISPR gene editing raises significant ethical implications, particularly surrounding who decides the appropriate uses of this powerful technology. Issues include the potential for ‘designer babies,’ health equity, and the morality of editing traits that do not represent disease. The ethical dilemmas highlight the need for comprehensive guidelines to ensure that CRISPR technology is used responsibly and justly.
How does CRISPR gene editing help in treating sickle cell anemia?
CRISPR gene editing provides a transformative approach to treating sickle cell anemia by allowing scientists to correct the genetic mutations responsible for the disease. This manipulation of somatic cells has led to successful treatments, potentially curing the disease for many patients. However, the high cost and accessibility of such treatments raise health equity concerns.
What are the potential risks of CRISPR gene editing technology?
While CRISPR gene editing technology offers remarkable potential, it also poses risks such as unintended genetic changes, ethical dilemmas, and the possibility of exacerbating health disparities. Scientists caution that any modifications could have unforeseen consequences, highlighting the need for careful oversight and ethical considerations.
What are the health equity concerns linked to gene editing technology?
Health equity concerns in gene editing technology, like CRISPR, primarily revolve around access to treatments. Innovations often benefit those with sufficient resources, potentially leaving marginalized groups without the necessary healthcare. Addressing these disparities is crucial to ensure that all individuals can benefit from advancements in gene therapy.
Can CRISPR address conditions that aren’t life-threatening?
CRISPR technology can technically be used to address non-life-threatening conditions, such as certain genetic traits or syndromes. However, this raises ethical questions about the appropriateness of editing human genes for enhancement purposes versus strictly medical reasons. The ongoing debate tackles how far gene editing should go in altering human genetics.
What oversight exists regarding CRISPR gene editing practices?
Currently, there are laws and guidelines against germline editing in many countries. However, oversight varies globally, with concerns about practices in countries with less stringent regulations. Effective monitoring of CRISPR gene editing is essential to prevent unethical applications, particularly in experimental treatments.
How does CRISPR gene editing differ from traditional gene therapy?
CRISPR gene editing differs from traditional gene therapy primarily in its precision and efficiency. While traditional therapies may involve inserting genes into cells, CRISPR allows for direct editing of existing genes, enabling more specific alterations. This advancement holds promise for curing genetic disorders with fewer side effects.
What are the implications of CRISPR for future generations?
The implications of CRISPR for future generations are profound. By editing germline cells, it is possible to eliminate hereditary diseases from the gene pool. However, this raises ethical questions about consent and the definition of normality, as future generations may face genetic modifications without their agreement. Discussions on these potential ramifications are crucial as the technology evolves.
| Key Points | Details |
|---|---|
| Ethical Dilemmas | The role of gene editing in altering human traits raises significant ethical questions, such as the implications of modifying abilities like hearing in children of deaf parents. |
| Health Equity Concerns | The high costs of CRISPR treatments, like the $2.2 million cure for sickle cell disease, highlight disparities in access to advanced medical treatments. |
| Technology Risks | Gene editing could lead to unintended consequences due to the complex interactions within genetic systems, as illustrated by the example of gene editing for LDL cholesterol. |
| Oversight Issues | Lack of global oversight raises concerns about the potential misuse of gene editing technologies in certain countries. |
| Cultural Perspectives | Diverse views on genetic conditions challenge the notion of ‘normal,’ emphasizing the value of human diversity instead of correcting perceived flaws. |
Summary
CRISPR gene editing represents a revolutionary breakthrough in biotechnology with the potential to combat genetic diseases. However, it also presents deep ethical questions concerning the risks of altering human genetics, including issues of health equity, access to treatment, and the right to make genetic modifications. As the conversation evolves, it is crucial to carefully balance innovation with ethical considerations, ensuring that advancements benefit all members of society.