Alzheimer’s Research: Transforming Treatments Through Science
Alzheimer’s research is at the forefront of today’s battle against neurodegenerative diseases, bringing new hope to millions suffering from cognitive decline. Pioneering work by scientists like Beth Stevens has illuminated the role of microglial cells within the brain’s immune system, reshaping our understanding of how these cells impact neuronal health. Stevens’ groundbreaking studies reveal that dysfunctional pruning by microglia may contribute to the onset of Alzheimer’s disease, paving the way for potential treatment breakthroughs. As the number of individuals diagnosed with Alzheimer’s in the U.S. continues to rise, the implications of this research are both crucial and urgent. By focusing on the mechanisms underlying Alzheimer’s, we inch closer to developing effective therapies and strategies that could fundamentally alter the course of this debilitating illness.
The ongoing exploration of Alzheimer’s disease encompasses a wide array of scientific inquiries into cognitive impairment and related disorders. In this realm, the study of brain immune responses—specifically through the lens of microglial cell function—plays a pivotal role in understanding how synaptic communication is affected in aging populations. Researchers like Beth Stevens are transforming our approach to neurodegenerative conditions by revealing how these immune cells interact with neuronal networks. With a focus on the molecular pathways involved, this line of inquiry demonstrates the critical relationship between immune function and the development of cognitive disorders. As we deepen our grasp of these biological processes, we open doors to innovative treatments that could alleviate the burden of Alzheimer’s on individuals and society.
Understanding Microglial Cells: The Brain’s Immune System
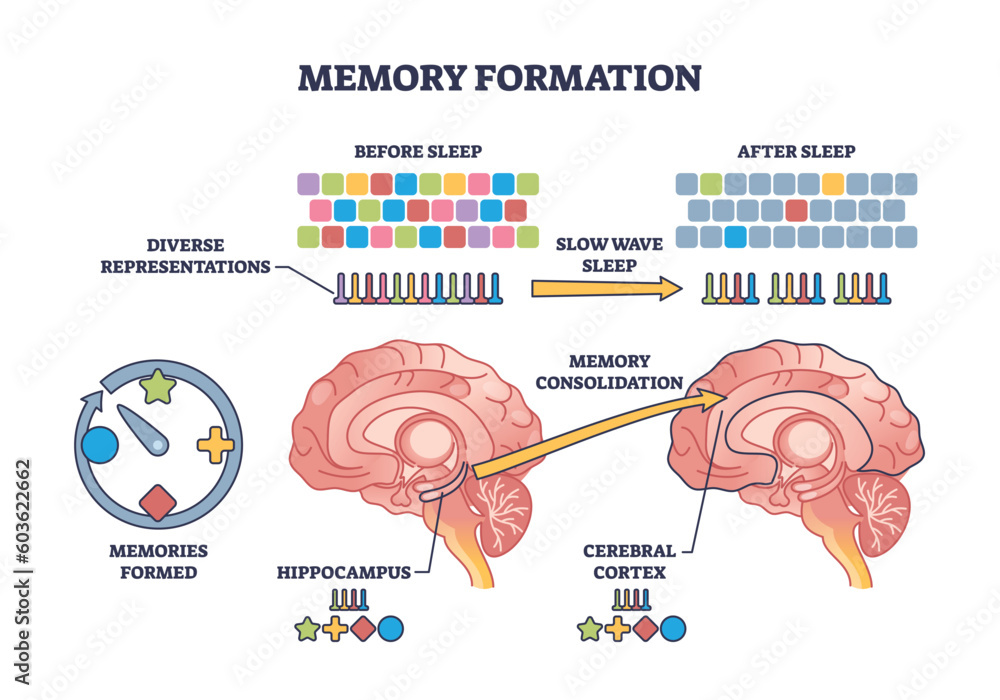
Microglial cells play a crucial role in maintaining the brain’s health. These specialized immune cells are responsible for monitoring the central nervous system for signs of distress, such as injury or disease. By clearing out damaged neurons and synapses, microglia ensure that the brain functions optimally. However, in the context of neurodegenerative diseases like Alzheimer’s, their function can become disrupted. Aberrant pruning conducted by microglia can lead to an accumulation of toxic proteins and inflammatory responses that exacerbate neuronal damage.
Beth Stevens’ groundbreaking research has unveiled the dual nature of microglial cells. While they are pivotal in protecting the brain, their misbehavior can lead to severe consequences, making them a focal point in Alzheimer’s research. Understanding the intricate mechanisms through which microglia interact with neurons not only reveals new insights into Alzheimer’s but also opens doors for novel therapeutic strategies aimed at fine-tuning microglial activity to support neuronal health and delay the progression of neurodegenerative diseases.
Alzheimer’s Treatment Breakthroughs: Innovations in Research
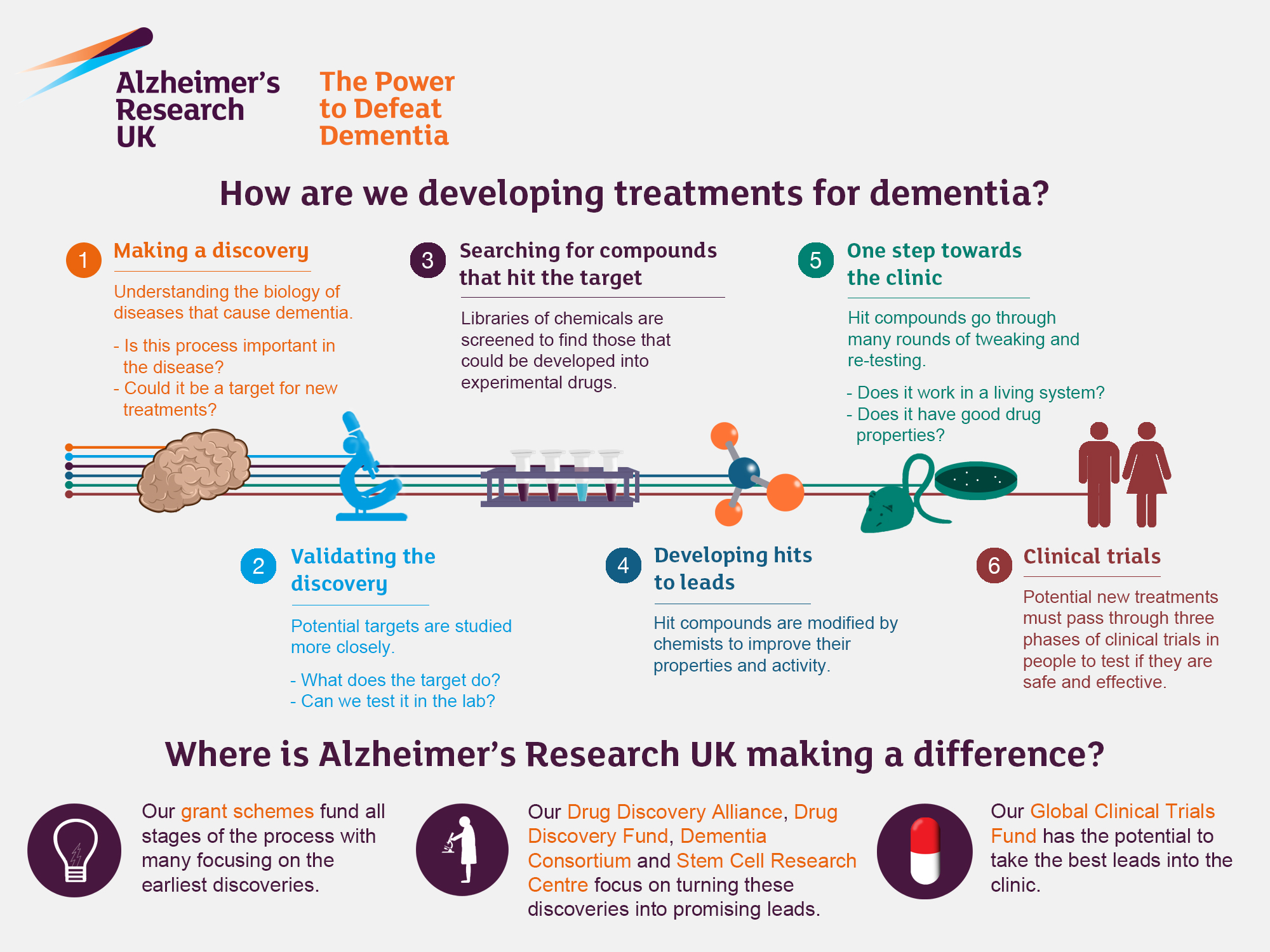
Recent advancements in Alzheimer’s treatment underscore a significant paradigm shift spurred by innovative research methodologies. The insights gleaned from studying microglial cells have paved the way for potential therapeutic interventions targeting the brain’s immune system. With the backing of organizations such as the National Institutes of Health, researchers are developing drugs that can modulate microglial function, attempting to reverse the detrimental pruning associated with Alzheimer’s pathology.
Furthermore, Stevens’ work emphasizes the importance of early detection through biomarkers associated with microglial activity. Identifying these markers can facilitate timely interventions, allowing treatment before significant neuronal damage occurs. The quest for Alzheimer’s treatment breakthroughs is bolstered by these scientific discoveries that not only promise improved outcomes for patients but also illuminate new pathways in the understanding of neurodegenerative disease processes.
The Future of Neurodegenerative Disease Research
Exploring the future of neurodegenerative disease research, especially Alzheimer’s, reflects an exciting intersection of science and hope. As the research landscape evolves, there is increasing recognition of the complexities involved in diseases like Alzheimer’s. With a growing emphasis on the role microglial cells play, ongoing studies are likely to illuminate further connections between immune mechanisms in the brain and neurodegeneration, potentially leading to breakthrough therapies.
Moreover, as the global population ages, the significance of this research becomes more pronounced. The anticipated doubling of Alzheimer’s cases by 2050 necessitates an urgent focus on innovative treatments and prevention strategies. By delving into the cellular processes influenced by microglia, researchers can identify actionable targets that could transform the management of Alzheimer’s and improve quality of life for millions affected by this devastating disease.
Funding and Support for Alzheimer’s Research Initiatives
The drive for advancements in Alzheimer’s research is deeply intertwined with funding and support from federal agencies. As highlighted by Beth Stevens, significant backing from institutions such as the National Institutes of Health has been essential for pioneering studies investigating microglial functions and their implications for neurodegenerative diseases. This financial support not only facilitates the exploration of scientific hypotheses but also bridges the gap between basic research and applied clinical outcomes.
With a strong foundation funded by critical government resources, the future of Alzheimer’s research holds promise for substantial discoveries. Ensuring continued investment in initiatives that examine the intersection of microglia and neurodegeneration will be vital in unlocking novel therapies. As researchers like Stevens rediscover the vital roles these brain immune cells play, the research community can harness these findings for transformative Alzheimer’s treatment breakthroughs.
The Role of Curiosity in Scientific Discoveries
Curiosity is often the driving force behind scientific breakthroughs, especially in complex fields like neuroscience. Beth Stevens reflects on her journey of discovery, emphasizing that her initial questions about the brain’s immune system led to impactful findings about microglial cells and their roles in neurodegenerative diseases. This journey highlights how fostering a culture of curiosity within scientific communities can lead to unexpected, yet vital, breakthroughs in understanding and treating Alzheimer’s.
Moreover, curiosity-driven research opens pathways to explore unconventional ideas, pushing boundaries of knowledge. By encouraging scientists to delve into areas that may seem far removed from clinical applications, we can uncover new frameworks that ultimately contribute to advancing Alzheimer’s treatment. Such explorations highlight the essential role that innovative thinking plays in translating basic research into tangible benefits for patients.
Identifying Biomarkers: Early Detection of Alzheimer’s Disease
Identifying reliable biomarkers is a crucial step toward early detection of Alzheimer’s disease, and recent research on microglial cells has brought us closer to achieving this goal. Biomarkers provide measurable indicators of the disease before significant cognitive decline occurs, offering a window of opportunity for intervention. By focusing on how microglia respond to emerging pathology, researchers like Stevens are developing assays that could revolutionize early diagnosis and management of Alzheimer’s.
The potential impact of these biomarkers extends beyond just early detection; they can also monitor disease progression and treatment response. As our understanding of the brain’s immune system deepens, it paves the way for personalized approaches to Alzheimer’s treatment that could adjust therapies based on individual biomarker profiles. Such innovations could ultimately lead to improved patient outcomes and a new era in the management of Alzheimer’s.
Public Awareness and Education on Neurodegenerative Diseases
Raising public awareness about neurodegenerative diseases, particularly Alzheimer’s, is a fundamental aspect of fostering understanding and support for ongoing research. Efforts to educate the population about the roles of microglial cells and the implications of their dysfunction can empower individuals to advocate for research funding and participate in clinical trials. Increased awareness can demystify the disease, dispel myths, and emphasize the importance of early detection and intervention.
Moreover, public education initiatives can play a pivotal role in encouraging individuals to be proactive about brain health. By promoting a better understanding of how lifestyle choices may influence neurodegenerative disease processes, we can collectively contribute to prevention efforts. As communities become more informed, the advocacy for Alzheimer’s research and the support of initiatives aimed at developing effective treatments will grow stronger.
The Importance of Interdisciplinary Approaches in Alzheimer’s Research
Alzheimer’s research is inherently complex, necessitating interdisciplinary approaches that combine insights from molecular biology, neuroscience, psychology, and immunology. Stevens’ work exemplifies how collaborative efforts among diverse scientific fields can drive innovation and foster comprehensive understanding. By uniting different perspectives, researchers can tackle multifaceted challenges posed by Alzheimer’s, integrating knowledge about microglial behavior, synaptic health, and neuroinflammation.
Interdisciplinary collaboration is crucial not only for scientific exploration but also for translating research findings into clinical practices. Combining expertise from various domains can lead to the development of holistic treatment strategies that address the full spectrum of Alzheimer’s pathology. As we continue to witness encouraging breakthroughs stemming from such collaborations, the future of Alzheimer’s care looks promising, highlighting the necessity of teamwork in scientific advancement.
Transforming Alzheimer’s Care: Promise of Research Innovations
The promise of research innovations in Alzheimer’s care is exemplified by the ongoing work in understanding microglial cells and their role in the disease. By elucidating the mechanisms that underlie Alzheimer’s pathology, scientists aim to develop targeted interventions that can alter disease trajectories. These research endeavors hold the potential to not only slow down the progression of the disease but also enhance the quality of life for millions living with Alzheimer’s.
Furthermore, as research translates into clinical practice, the goal is to create a framework that supports both patients and caregivers. Innovations in Alzheimer’s care, driven by scientific discoveries, can empower families with effective management strategies and resources for navigating the complexities of dementia. Ultimately, the collaborative efforts in Alzheimer’s research promise to transform the landscape of care and offer hope for a future where Alzheimer’s is not a debilitating reality.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they act as the brain’s immune system. They are responsible for patrolling the brain, clearing away damaged cells, and pruning synapses, which are vital for neuronal communication. Aberrant pruning by microglia has been linked to Alzheimer’s disease and other neurodegenerative disorders, making them a key focus of ongoing research.
How are microglial cells related to neurodegenerative diseases like Alzheimer’s?
Microglial cells are implicated in neurodegenerative diseases such as Alzheimer’s due to their role in maintaining brain health. In Alzheimer’s research, scientists investigate how dysfunctional microglial activity can lead to the accumulation of toxic proteins and synaptic damage, thereby contributing to disease progression.
What advancements have been made in Alzheimer’s treatment breakthroughs involving microglial cells?
Recent breakthroughs in Alzheimer’s treatment involve targeting microglial cells to enhance their protective functions. Research led by scientists like Beth Stevens has highlighted how correcting abnormal microglial activity could lead to new therapeutic strategies for Alzheimer’s, potentially slowing down or halting the disease.
How do microglial cells contribute to the identification of early biomarkers for Alzheimer’s disease?
Microglial cells are pivotal in Alzheimer’s research for identifying early biomarkers. Studies have shown that changes in microglial behavior can precede visible symptoms of Alzheimer’s, providing a basis for developing biomarkers that could help in the early detection of the disease, thus improving patient outcomes.
What is Beth Stevens’ contribution to Alzheimer’s research?
Beth Stevens has made significant contributions to Alzheimer’s research by advancing our understanding of microglial cells and their role in synaptic pruning. Her work has led to insights into how dysfunctional microglial activity can contribute to neurodegenerative diseases, influencing the development of new therapeutic approaches and potential treatment breakthroughs.
What is the impact of neuroinflammation in Alzheimer’s disease according to recent research?
Recent research in Alzheimer’s disease highlights the impact of neuroinflammation, primarily mediated by microglial cells. Chronic activation of microglia can lead to a harmful inflammatory response that exacerbates neuronal damage and promotes disease progression, making it an important target in Alzheimer’s research.
How does the brain’s immune system relate to Alzheimer’s disease?
The brain’s immune system, primarily mediated by microglial cells, plays a crucial role in Alzheimer’s disease. Healthy microglial function helps protect against neurodegeneration, while dysfunctional microglia can accelerate disease processes, underscoring the need for research aimed at understanding and correcting these cellular pathways.
What future directions are being explored in Alzheimer’s research related to microglial cells?
Future directions in Alzheimer’s research focus on manipulating microglial function to enhance their protective roles, developing targeted therapies that could restore normal pruning processes, and identifying biomarkers related to microglial activation that could facilitate earlier diagnosis and intervention in Alzheimer’s disease.
| Key Point | Details |
|---|---|
| Research Focus | Beth Stevens explores the role of microglial cells in the brain, which act as the brain’s immune system. |
| Microglial Function | Microglia patrol the brain, clearing dead cells and pruning synapses to maintain brain health. |
| Contribution to Disease | Aberrant pruning by microglia is linked to Alzheimer’s disease and other neurodegenerative disorders. |
| Impact of Research | The research offers insights into potential treatments and early biomarkers for Alzheimer’s disease. |
| Funding Importance | Vital federal funding has supported the research, enabling significant scientific advancements. |
| Growth of the Problem | The number of Alzheimer’s cases is expected to double by 2050, stressing the need for innovative research. |
Summary
Alzheimer’s research has taken significant strides behind the efforts of dedicated scientists like Beth Stevens, who emphasizes the importance of understanding microglial cells in the brain’s immune response. Stevens’ findings reveal that the malfunctioning of these cells contributes to Alzheimer’s disease, highlighting the need for further exploration into their role. As we face a projected doubling of Alzheimer’s cases by 2050, the ongoing research not only promises to revolutionize treatment options but also aids in the early detection of this debilitating disease, showcasing the critical connection between basic science and real-world medical applications.